Studies & Collaborations

Your competence center for Clinical trials, Performance studies and collaboration
The Studies & Collaborations team is your contact for industry collaborations, clinical trials, and performance evaluations. Our services range from contract analysis to prototype development and prototype testing. With a shared vision for a better future, we forge new paths, tackle medical challenges, and explore new possibilities for diagnostics.
Send request
Studies
- Our parent companies Charité and Vivantes work with thousands of scientists and doctors on pioneering developments in medical research. Consequently, Charité Berlin, as a university hospital, conducts numerous studies. Acting as an interface between laboratory diagnostics and the clinic, we deliver various diagnostic parameters to support researchers investigating scientific hypotheses.
- As an interface between laboratory diagnostics and the clinic, our department supports researchers with scientific questions on the determination of various parameters.
For specific contract analyses feel free to browse our services directory.
to the list of services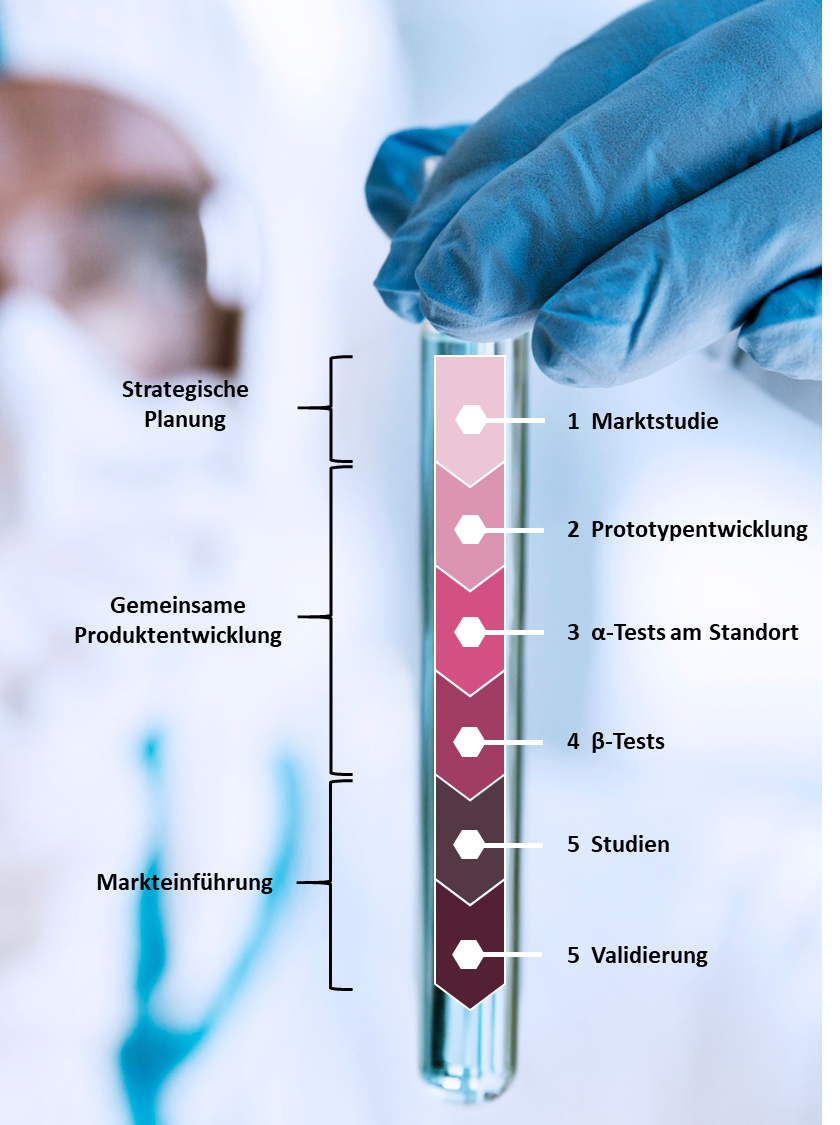
©
©Labor Berlin
Collaborations
- We have long-standing experience in collaborating with the top players from the in-vitro diagnostics and pharmaceutical industries.
- Our team is specialized in the planning, execution, and documentation of studies. It possesses extensive knowledge of the regulations according to AMG/CTR and MPDG/IVDR.
- The international ethical and scientific quality standard “Good Clinical Practice (GCP)” according to DIN EN ISO 20916 or ICH GCP E6 is a given for us.
- Due to the recently introduced IVDR 2017/746 requirements, the need for performance studies and market surveillance studies has increased. We are convinced that the development of new in-vitro diagnostics will be significantly improved by early feedback from intended users in the laboratory environment and access to clinical samples.