Autoimmune Diagnostics
The Department of Autoimmune Diagnostics emerged from several individual laboratories at Charité and Vivantes.
Autoimmune diagnostics services today include
- the entire laboratory diagnostics of patients with autoimmune diseases using state-of-the-art laboratory and immunodiagnostics,
- the support of publicly funded third-party projects and
- participation in industry-sponsored and investigator-initiated studies.
-
Autoimmune diagnostics currently determines around 140 parameters using the following methods:
- Immunofluorescence
- ELISA
- Immunoblot
- PCR
- Microarray
The equipment includes all devices required for parameter determination, for example:
- Immunofluorescence microscopy (IF)
- Automatic preparation of slides for IF microscopy
- Aklides (for electronic evaluation of IF slides)
- Microplate reader
- Laboratory machines
- ELISA
- Blot-Master
- Blot-Scanner
- PCR-Cycler
- Microarray-Scanner
-
Interpretation of the measurement results
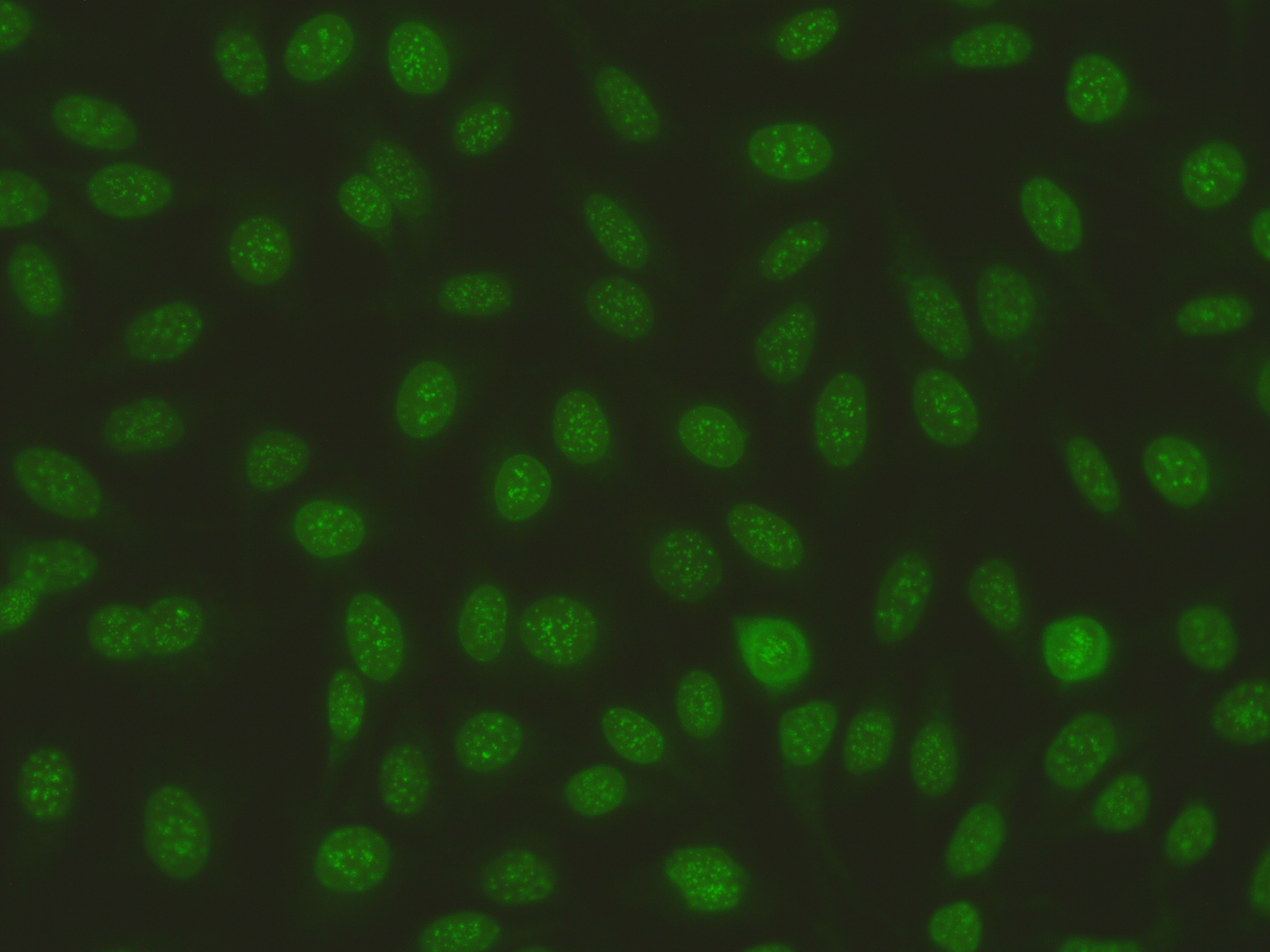
The principle of immunological detection techniques used in autoimmune diagnostics is the antigen-antibody reaction in which antigen (Ag) and antibody (Ak) form a specific, reversible bond. The methods currently used in autoimmune diagnostics include indirect immunofluorescence (IIFT), enzyme-linked immunoassay (EIA) and immunoblotting/line immunoassay (LIA).
Indirect immunofluorescence on HEp-2 cells (HEp-2 IFT)
(Previously: antinuclear autoantibodies, ANA)
The determination of autoantibodies using indirect immunofluorescence (IFT) is an essential part of the diagnosis and classification of a number of autoimmune diseases. Apart from the qualitative statement “negative” or “positive”, this study provides further valuable information. In addition to the intensity of the reaction, which is caused by the amount of antibodies present and represented as a titer, the autoantibodies can cause a variety of fluorescence patterns relating to the nucleus, the cytoplasm and the mitotic stages of the cells examined.
HEp-2 cells are used as a substrate for screening for organ-nonspecific autoantibodies. The test results can sometimes vary, both between individual employees of a laboratory and between different laboratories. The causes of these deviations can be objective, e.g. different reagents, or subjective, due to different empirical values.
The “ICAP” initiative (International Consensus on Antinuclear Antibody Patterns (ANA)) was founded with the aim of finding a uniform nomenclature for various fluorescence patterns that can be recognized and described using the indirect immunofluorescence technique on HEp-2 cells. As this classification system includes nuclear, mitotic and cytoplasmic antibodies, the term “HEp-2 cell IFT” is recommended for the screening test.
Further detailed information on the classification and nomenclature is available under the following link:
The clinical relevance of the detected autoantibodies should always be assessed in the context of the suspected clinical diagnosis and usually confirmed or supplemented by another method.
Literature:
E.K.L. Chan, J. Damoiseaux, O.G. Carballo, K. Conrad, W. de Melo Cruvinel, P.L.C. Francescantonio, M.J. Fritzler, I. Garcia-De La Torre, M. Herold, T. Mimori, M. Satoh, C.A. von Mühlen, and L.E.C. Andrade. Report of the First International Consensus on Standardized Nomenclature of Antinuclear Antibody HEp-2 Cell Patterns (ICAP) 2014-2015 (Front. Immunol. 2015, Aug 20;6:412).
Enzyme-linked Immunoassay (EIA)
Usually more sensitive than IIFT, which may be associated with a loss of specificity. Results may differ from those determined by another method. The significance of the different methods and their results must always be assessed with the clinical picture of the patient, as the structure or presentation of the antigen can vary individually depending on the method, as can the time course and level of antibodies, or their fall and rise.
Immunoblotting, Line Immunoassay (LIA)
This method can be helpful if several antibodies are to be detected simultaneously, e.g. onconeuronal antibodies. The results obtained with this method are quantitative or semi-quantitative:
– negative(+) questionable/limited
+ weakly positive
++ positive
+++ strongly positive
In principle, a higher sensitivity of the laboratory tests also means a higher diagnostic sensitivity, but at the same time a lower specificity. The evaluation must take into account that there is currently no gold standard method in this area of diagnostics. The lack of standardization of methods can cause different results in different laboratories and large differences in the implementation of external quality controls (interlaboratory comparisons). Furthermore, the individual methods are not comparable in terms of their informative value.
The critical difference, which depends on the precision of the method (standard deviation, SD), is of great importance for progression assessment. The difference between two determinations can be described as statistically significant if two measurement results differ by at least +3SD.
In summary, the use of different methods, which can complement each other, is necessary for diagnosis. An effective and successful diagnosis can only be achieved with the help of a link between patient, doctor and laboratory, whereby the clinical manifestation of the disease plays an essential role in the interpretation of the laboratory results.
Literature:
K. Dörner: Klinische Chemie und Hämatologie, 2009
A. Hall et al.: Clinical Immunology, Fundamental of Biomedical Science, 2016
B. Neumeister, B. O. Böhm: Klinikleitfaden: Labordiagnostik, 2015
K. M. Pollard: Autoantibodies and Autoimmunity, 2006
G. Spickett: Oxford Handbook of Clinical Immunology and allergy, 2020contact: autoimmundiagnostik@laborberlin.com
-
Integrated in research
The Department of Autoimmune Diagnostics is involved in numerous research projects in the clinical and industrial sectors. New important assays for the determination of autoantibodies have been developed as part of these projects, for example:
- Anti-MCV-ELISA
- POCT-Assay RheumaChec
- Anti-dsDNA-ELISA
- Anti-PR3-hn-hr-ELISA
- Epitope mapping for therapy monitoring of biologicals in rheumatoid arthritis (RA)
In the national multi-center study Hit-Hard, which was initiated by the Medical Clinic with a focus on rheumatology and clinical immunology, the reference laboratory. The laboratory is also part of two joint research projects and contributes to numerous therapy studies.
Requisition slips
Services for private patients, self-paying patients or elective laboratory services will be invoiced by LABOR BERLIN directly to the respective payer, unless otherwise agreed with the sender. For this purpose, the sender shall forward the necessary patient data to LABOR BERLIN and ensure that the patients are informed about the possible forwarding of laboratory orders to LABOR BERLIN and the associated organizational measures, including billing by a private medical clearing office, in the manner prescribed by law and consent to this. The legal requirements with regard to the free choice of doctor are taken into account. We would like to point out that, in accordance with the provisions of the German Hospital Remuneration Act (KHEntgG), external elective laboratory services must be ordered by the sender on a case-by-case basis and specifically by the elective physicians concerned.
-
Requisition slip for autoimmune diagnostics
pdf





