Studien, Kooperationen & Innovationsmanagement

We believe IVDR drives the need for performance studies and market surveillance studies in a clinical laboratory setting. We believe better products can be developed if creators of future IVDs get early feedback from end-users and lean access to clinical samples.
Our customers:
- In-vitro diagnostics industry (IVD)
- Contract research organization (CRO)
- Research institutions (RUO testing)
- Pharmaceutical industry
- Life science companies
- Our offerings
- Study implementation in a variable testing environments (core lab, satellite lab, POCT setting)
- Study design support
- Compilation of IRB application, contact point for all queries for the IRB
- Co-development of new biomarkers with industrial collaboration partners
- Reference method development
- Performance study services in a clinical laboratory setting
- Bio statistical analysis
- Result presentations
-
- Dedicated project leaders, research scientists and technicians for your project
- Dedicated lab space with different levels of confidentiality
- Recruitment of healthy volunteer and patient groups
- Full spectrum of laboratory diagnostics across 9 diagnostic fields
- Compliance with the current quality specifications
- ISO 20916 compliant study environment
- ISO 15189 accredited laboratory
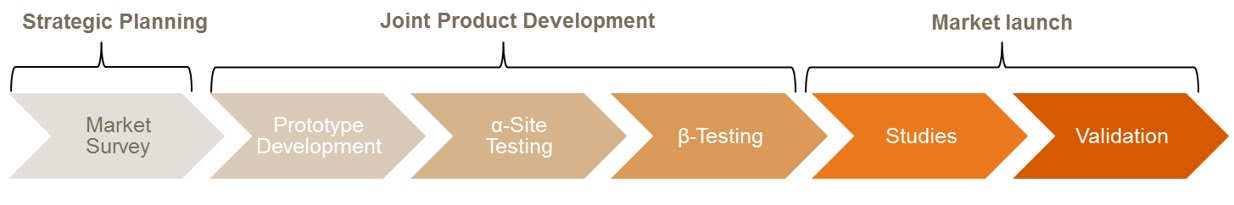
Common types of studies and collaborations
-
Lean and fast access to clinical samples to guide product development planning and planning of performance studies.
-
System requirements and specifications, assay development, clinical and laboratory assessment, comparative evaluation of novel diagnostic platforms to current methods (“gold standard”), Center of Excellence.
-
PD- and PK-assessments, patient screening, study-specific routine testing.
-
Access to clinical experts and end-users of IVDs to deliver input e.g. for design control processes and access to real world data on throughput, clinical performance of current test, cost-structures and reimbursement to help building business models and defining benchmarks for new products.

